
I just read about scientists using CRISPR to fix a gene in human embryos. Is this a big deal?
August 8, 2017

- Related Topics:
- Bioethics,
- CRISPR,
- Gene therapy,
- Genetic engineering,
- Futuristic science,
- Genetics in the news
A curious adult from California asks:
"I just read about scientists in Oregon using CRISPR to fix a broken gene in human embryos. Is this as big a deal as the news stories are making it out to be?"
It is a big deal but probably not as big as some media outlets would have you believe (or believe themselves).
It is big because this is the first time that scientists have used the gene editing tool CRISPR/Cas9 to specifically change a gene in a human embryo here in the US. This is a very big step.
It is also important because the researchers learned some things about gene editing in embryos that will make it easier and more reliable in the future.
Past attempts (like the one here) have resulted in embryos where some cells had their DNA repaired and some didn’t. If these embryos were put into mom and born nine months later, their genetic disease would almost certainly not be cured. At the very least the cure would not be a sure thing.
These “mosaics” (as people with mixed cells like this are called) did not happen in this study. In most of the embryos, either all of the cells had the fixed DNA or none of them did. This has been rightly celebrated as a very important finding in this study that paves the way for future gene editing.
The scientists also learned that embryos are way better at repairing their DNA than are the other cells that have been tested in the past. Some of the tricks scientists have had to use can be left out which makes future work simpler.
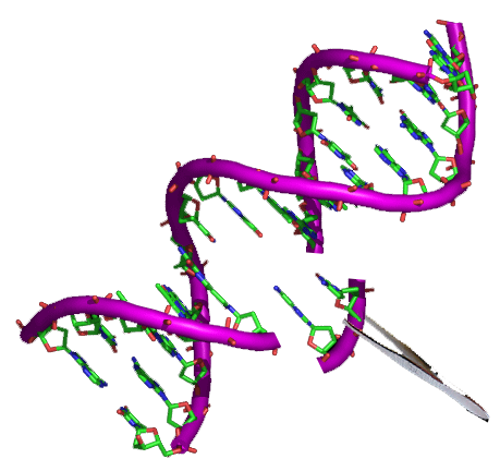
Exaggerated reporting?
Other parts of the story aren’t always as impressive as they were sometimes reported though. (The scientists themselves did not make any overblown claims.)
For example, some stories make a big deal of the fact that after the treatment, 72% of the embryos in the experiment did not have the damaged gene. What they leave out is that even without gene editing, this would have been true for half of them anyway.
That means the real number of repaired embryos is more on the order of 22%. Impressive but not nearly as impressive as 72%.
That 72% number brings up another issue—is this gene editing better than what we have available now? Is that boost from 50% to 72% worth the unknown risks of gene editing?
For the genetic disease they worked on, the answer is no. There are perfectly good techniques like preimplantation genetic diagnosis (PGD) available that could ensure that parents would not have a child with the disease without having to tinker with the DNA of an embryo.
All the steps are the same with the two techniques right up to the gene editing. The difference is that instead of changing the embryo’s DNA, with PGD scientists select out the embryos that did not inherit the damaged gene. Only embryos that will not end up with the genetic disease are put into mom.
So PGD will probably be better than gene editing for most cases. But not all of them. There is a smaller subset of cases where gene editing is the only way some parents could have a healthy child.
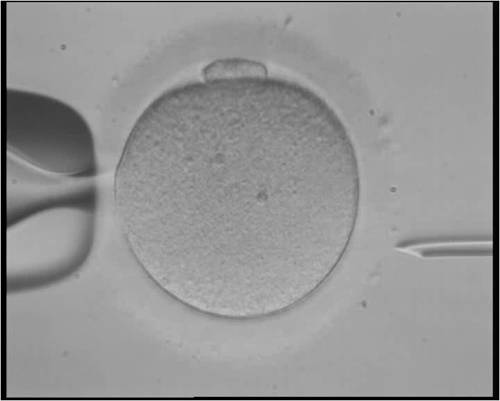
One situation where gene editing might be an important add-on is in cases where the parents make very few eggs or sperm. In these cases it might be important to boost the number of fertilized eggs that have an undamaged gene.
Another case would be when each child has a 100% chance of ending up with the DNA that leads to a genetic disease. This would be the case, for example, if both parents have sickle cell anemia. Each child would end up with it too unless the DNA was corrected in the embryo.
The bottom line here is that at least for now and probably into at least the near future, gene editing in embryos will be helpful for a small subset of the already small group at risk for passing on a significant genetic disease. To be more widely useful the gene editing will have to become more efficient both in how many embryos have repaired genes and how many genes are repaired per embryo.
We may be one step closer to curing genetic diseases but there are many steps to go. A journey of a thousand miles may begin with a single step, but you still have to go that thousand miles.
Gene Editing in an Embryo
In the past, the CRISPR gene editing system has needed three things to edit a gene:
- Cas9: The part that cuts the DNA
- Guide RNA (gRNA): The part that gets Cas9 to the right spot in the DNA to cut
- Homologous DNA: The corrected version of the DNA that is used by the cell to fix the damaged gene
Basically, the way it worked previously was that scientists would first use in vitro fertilization (IVF), fertilizing eggs with sperm in a dish. Then scientists would inject all three parts of the CRISPR system into the fertilized egg.
The idea is that the CRISPR system can now get to work on the fertilized egg’s DNA. Done this way, you inevitably ended up with an embryo with some cells that have the changed DNA and some that don’t.
These “mosaic” embryos would probably grow into babies that still have the disease. To cure the disease, we need most if not all of the embryo’s cells to have their broken gene repaired.
The scientists in this most recent study solved this “mosaic” problem by doing a couple of things differently. Instead of a fertilized egg, they injected a sperm cell along with all three parts of the CRISPR system into an egg. In this case fertilization was happening at the same time as the CRISPR system was getting to work on the DNA.
When these scientists did this they found that they no longer got mosaics. Now either all of the cells had their DNA repaired or none of them did. As I said before, this is a really big deal and an incredibly important finding.
They also found that they didn’t need the third part of the system—the homologous DNA. The embryos instead used their own DNA to fix their DNA.
While this does simplify things because scientists can get away with adding just two parts of the CRISPR system, it might make some editing tricky. Particularly if scientists need to correct both copies of a gene.
Two Copies: More than Twice as Hard?
We have two copies of most of our genes. We get one from mom and one from dad.
In this study, they worked on a disease where only one of the copies needs to be damaged to cause a disease. This is called a “dominant” genetic disease.
In this case, Cas9 cut the damaged copy but left the working one alone. The embryo the used its second working copy to fix its broken gene. It ignored the DNA the scientists added.
This is great in cases where there is a working copy of a gene but what if we are dealing with a disease with two broken copies? A “recessive” genetic condition?
In this case, Cas9 might cut one but then the other equally broken copy might be used to “repair” the broken one. The end result is the same two broken genes we started with!
Or Cas9 might cut both and then be forced to use the added DNA. Scientists will need to do more work to figure out how efficient this would be.
This matters because it could directly affect a subset of patients for whom gene editing would be useful—parents who both have a recessive disease.
In a recessive disease, both gene copies are broken. And if both parents have the same recessive disease, then all of their children will probably end up with it too. Both parents will pass a broken gene down to their child.
In these situations, gene editing could prevent the child from inheriting their parents’ disease. If the embryo can be coaxed to use the added DNA instead of its own DNA that is.
As you can see, there is still a lot and I mean a lot of work left to do. These results are encouraging but don’t expect miracle cures any time soon.
Well, not any more miraculous than we can already get with some amazing techniques like preimplantation genetic diagnosis (PGD).

Author: Dr. D. Barry Starr
Barry served as The Tech Geneticist from 2002-2018. He founded Ask-a-Geneticist, answered thousands of questions submitted by people from all around the world, and oversaw and edited all articles published during his tenure. AAG is part of the Stanford at The Tech program, which brings Stanford scientists to The Tech to answer questions for this site, as well as to run science activities with visitors at The Tech Interactive in downtown San Jose.
 Skip Navigation
Skip Navigation
